Clinical trials point the way forward
November 03, 2016
Doctors at UCI Health are
investigating an exciting new
medication that uses a patient’s own
immune system to attack leukemia cells.
Led by Dr. Susan O’Brien, one of the nation’s
foremost leukemia experts and medical
director of The Sue and Ralph Stern Center
for Cancer Clinical Trials and Research, the
study is being conducted at only six centers.
UC Irvine Medical Center is the only site in
Southern California to test this novel agent
for chronic lymphocytic leukemia (CLL).
“As a National Cancer Institute-designated
comprehensive cancer center, it’s one of our
missions to conduct research,” says O’Brien,
who was involved in developing four new
CLL drugs approved by the Food and Drug
Administration in the past three years. “It’s
also our duty to foster improvements in the
excellent care we deliver today by discovering
even more effective treatments.”
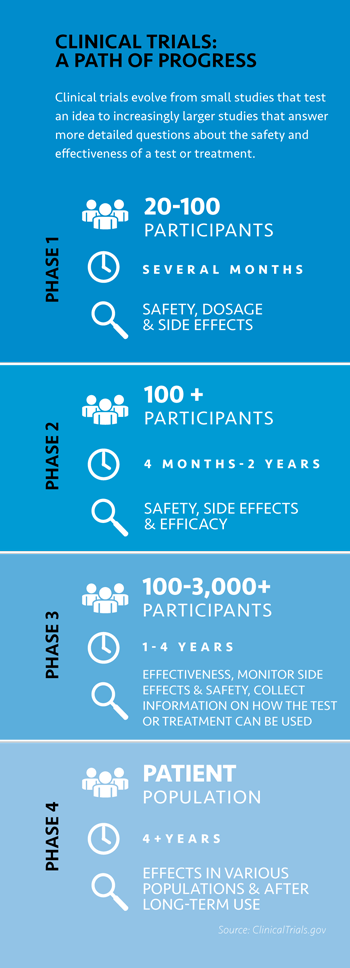
The medication
to treat CLL is
one of many
investigations
underway at UC
Irvine Health. It’s
a Phase 1 clinical
trial — the first
of a three-part
series of studies
required for new
drugs to be approved for treatment of cancer
or other conditions. These early studies
can often be described as “translational
medicine.” That means promising studies in
research laboratories are used or “translated”
to enhance human health. This bench-tobedside
approach accelerates the pace of
new cancer treatments, O’Brien says.
Participating in a clinical trial may offer hope
for patients with cancers that haven’t responded
to the standard of care. If an investigational
drug turns out to be effective, participants in the
trial will have benefited from it long before it’s
available to patients everywhere.
The FDA requires that clinical trials assess
the risk of side effects. Patients enrolled in
clinical trials receive close monitoring for
side effects, as well as an assessment of their
response by experts in their particular cancer.
“There have been a number of
publications that report patients in clinical
trials get excellent care because of the close
monitoring,” O’Brien says.
Still, some patients fear they might be
given a placebo if they participate in a clinical
trial. It’s a common misconception, O’Brien
says, noting that in cancer trials placebos
are almost never used. In early trial phases,
patients often receive an investigational
drug along with the standard of care. Even in
Phase 3 trials, where patients are randomly
assigned to treatments, many designs give
all patients the standard of care while some
patients receive that care plus the new drug.
For example, a large randomized trial
led by UCI Health Chao Family
Comprehensive Cancer Center investigated
the effectiveness of chemotherapy alone
or chemotherapy plus bevacizumab for
treatment of advanced cervical cancer. The
results of the Phase 3 clinical trial, published
in 2014 in The New England Journal of
Medicine, showed significantly longer
survival for those patients who received
chemotherapy combined with the new drug
and led to approval of the drug.
“It was a very important study that
changed the practice of how we treat
advanced cervical cancer,” O’Brien says.
UCI Health physicians often lead
national clinical trials or initiate their own
research projects. They conduct research into
many types of cancers and lead clinical trials in
Phases 1 through 3. O’Brien expects to launch
a program next year to expand the scope of
Phase 1 trials. The goal is to provide earlier
access to new drugs for patients who’ve failed
to respond to the standard therapies.
“We want to increase our clinical trials
to provide our patients with the most
promising options right here in Southern
California,” she says.
Learn more about clinical trials at ucirvinehealth.org/clinicaltrial.
— UCI Health Marketing & Communications
Featured in UCI Health Live Well Magazine Fall 2016
